FCM EC 1935/2004
The FCM EC Regulation 1935/2004 requires that FCMs must not release ingredients into food in quantities of, that could endanger human health or cause compositional changes, scent or taste foods. All FCMs must be made in accordance with with the principles of good manufacturing practice set out in the regulation (ES) No. 2023/2006.
Europe has aharmonised legal framework for FCM security.
Some FCMs, such as plastics, ceramics and regenerated cellulose, are covered by independent u EU-wide rules. They shall be used as a means of demonstrating compliance with the general safety requirements of the Framework URedbe.
All food contact materials , subject to a specific ucrepe in Europe, is to be be attached to in writing a declaration o compliance. It must state, to comply with the rules, that apply to them. In addition to of this is to be to be provided by Support documentation and Traceability z by marking or documentation v Delivery chains.
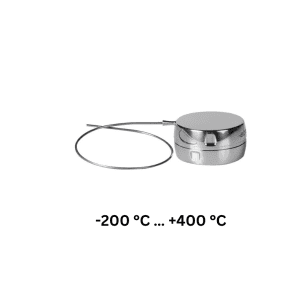
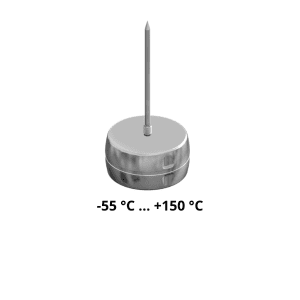
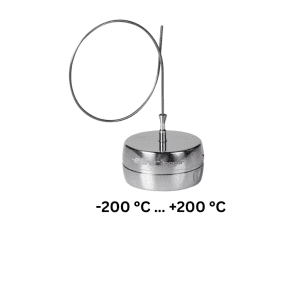
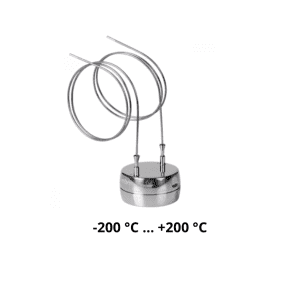
Introducing Ebro data loggers to ensure your compliance with the Regulation
FCM EC 1935/2004
.
Filter
Showing 1–40 of 41 results